

These cylinders were closed except for two small holes, one in each cylinder, placed so that the cathode rays could pass through them into the inside of the inner cylinder. This has been proved to be the case by Perrin, who placed in front of a plane cathode two coaxial metallic cylinders which were insulated from each other: the outer of these cylinders was connected with the earth, the inner with a gold-leaf electroscope. If these rays are negatively electrified particles, then when they enter an enclosure they ought to carry into it a charge of negative electricity. The following experiments were made to test some of the consequences of the electrified-particle theory. The electrified-particle theory has for purposes of research a great advantage over the aetherial theory, since it is definite and its consequences can be predicted with the aetherial theory it is impossible to predict what will happen under any given circumstances, as on this theory we are dealing with hitherto unobserved phenomena in the aether, of whose laws we are ignorant.

It would seem at first sight that it ought not to be difficult to discriminate between views so different, yet experience shows that this is not the case, as amongst the physicists who have most deeply studied the subject can be found supporters of either theory. The most diverse opinions are held as to these rays according to the almost unanimous opinion of German physicists they are due to some process in the aether to which-inasmuch as in a uniform magnetic field their course is circular and not rectilinear-no phenomenon hitherto observed is analogous: another view of these rays is that, so far from being wholly aetherial, they are in fact wholly material, and that they mark the paths of particles of matter charged with negative electricity. The experiments * discussed in this paper were undertaken in the hope of gaining some information as to the nature of the Cathode Rays. Is exploited in making powerful tools for the exploration of the atomic world, like the mass spectrometer.J. The force that moves electrons through the vacuum tube is an essential force that mediates all important interactions in chemistry. The cathode rays that impact the butterfly cause it to fluoresce, just as Thomson's screen was made to fluoresce in his classic experiment. Within the tube is a butterfly that is painted with fluorescent compounds. In our cathode ray tube, we run electrons - or cathode rays - through the tube. The cathode sprayed out its "rays" and those not absorbed by the anode would illuminate the screen, leaving a shadow of the cross-shaped. Beyond the anode was a fluorescent screen covered with zinc sulfide. Thomson (1856-1940) the electrons were introduced at one end containing the cathode and collected in the middle by a cross-shaped anode.

In between, the rays encounter a fluorescent screen, causing it to glow. The charged electrons in the electric field feel a force that causes them to flow from the cathode toward the anode where many are captured. The voltage placed across the tube creates an electric field.
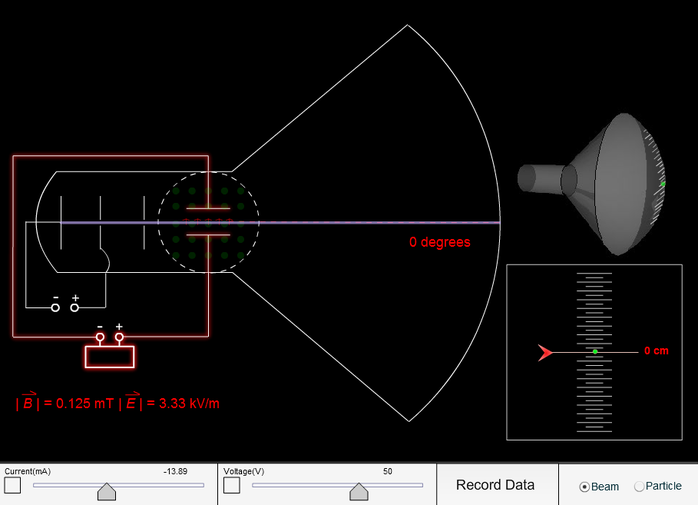
The electrons are introduced to the vacuum tube by the cathode. The mysterious "cathode rays" flowing through the vacuum tubes are now known to be currents of electrons. Apply voltage to end of cathode ray tube. Voltage is applied across a vacuum tube creating an invisible beam of "cathode rays" that magically illuminate a fluorescent screen.ġ.


 0 kommentar(er)
0 kommentar(er)
